What we can learn from squabbling bats
Patricia Jones
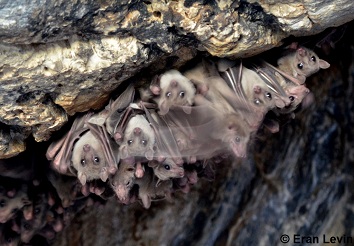
Egyptian fruit bat, Rousettus aegyptiacus. Photo by Eran Levin.
This week's paper is in Scientific Reports and comes from Yossi Yovel's group at Tel Aviv University, lead authored by Yosef Prat. If you have spent any time around bats, you are well aware that they do a lot of chattering. The Yovel group tackled the question of what is all that chattering about? To do this they video and audio recorded the behavior of 7 female Egyptian fruit bats, Rousettus aegyptiacus, (in a group of 22) over 75 days, totaling almost 15,000 individual vocalizations. They then used a machine learning approach to assess variation and information in bat calls. They were able to identify calling individuals with 71% accuracy, indicating that bats have enough variation in their calls to tell them apart. Now it starts to get really cool. Not only could the machine learning identify which individuals were calling, it could also detect differences in whom they were addressing. That is bats make slightly different calls when they are addressing different bats. Some of this variation is due to sex, they make different calls when they are talking to males versus to females, but also they are making slightly different calls when talking to different individuals within a sex. This means that an eavesdropping bat could potentially tell not only which bat is talking, but also which bat they are addressing. What the authors don't clarify is how repeatable these individual differences are across vocalizing bats. That is, are all of the bats referring to an individual using particularly vocal variants, like a name? Or do different bats use slightly different "names" for each of their roostmates? The authors notes that there was one bat who did not vary in their vocalizations depending on whom they were addressing. That one jerk who is yelling indiscriminately at everybody.
The authors then looked at the contexts in which bats were making vocalizations and showed that bats make different calls in different types of arguments (apparently Egyptian fruit bats mostly argue). The vocalizations they make are different when squabbling about food, or sleep space, or when somebody is making unwelcome sexual advances. The authors are also able to predict from the vocalizations what the outcome of the squabble will be. It would be great to know more about this, are the calls of the bats who win the squabbles louder, deeper, more chaotic?
Regardless of my desire for more detail, this shows how much can be gained just from making lots and lots of careful observations.


!["Figure 1. Training and testing protocol for associative learning in pea seedlings. (A) During training seedlings were exposed to the fan [F] and light [L] on either the same arm (i) or on the opposite arm (ii) of the Y-maze. The fan served as the c…](https://images.squarespace-cdn.com/content/v1/53d66e57e4b021e113e6c4c9/1481307094035-LBRG6LTW1S1QU1NWX7SI/image-asset.png)